Basic Fluid Properties in Fluid Mechanics
Fluids, which include both liquids and gases, are fundamental in fluid mechanics, where their behavior is analyzed based on key properties that influence motion, pressure, and flow dynamics. These properties determine how fluids respond to external forces and interact with their surroundings in various engineering applications.
Basic Fluid Properties
Overview of Formulas
| Density | |
| Specific Value | |
| Specific Weight | |
| Specific Gravity | |
| Bulk Modulus of Elasticity | |
| Compressibility | |
| Dynamic Viscosity | |
| Kinematic Viscosity | |
| Surface Tension | |
| Capillary Action | |
1. Density
Density is the ratio of mass of fluid and its volume. It represents how much matter is packed into a given space and is one of the most fundamental fluid properties.
![]()
Where:![]()
![]()
![]()
Density determines the inertial characteristics of a fluid. Higher density fluids require more force to accelerate and carry more momentum. Density is temperature and pressure dependent, fluids generally become less dense as temperature increases and more dense as pressure increases.
Density is crucial for calculating fluid forces, buoyancy, pressure distributions, and mass flow rates in piping systems.
Typical Values
| Fluid | Density ( |
| Water | 998 |
| Air | 1.2 |
| Mercury | 13560 |
| Glycerin | 1260 |
Example
The glycerin has a mass of 1200 kg and a volume of 0.952 cu.m. Find its density?
![]()
![]()
![]()
2. Specific Volume
Specific volume occupied by a unit mass of fluid. It is the reciprocal of density and is particularly useful in thermodynamic analysis.
![]()
where:![]()
![]()
![]()
![]()
While density tells us how much mass fits in a volume, specific volume tells us how much space a given mass occupies. This property is particularly useful in thermodynamics and when analyzing gas behavior.
3. Specific Weight
Specific weight (also called unit weight) is the weight of fluid per unit volume. It represents the gravitational force exerted by a fluid per unit volume.
![]()
Where:![]()
![]()
![]()
![]()
![]()
Specific weight directly relates to hydrostatic pressure. It tells us how much gravitational force acts on a volume of fluid, making it essential for pressure calculations in static fluids.
Example
Specific Gravity of an oil is 0.82. Determine its specific weight
![]()
![]()
![]()
4. Specific Gravity
Specific gravity (relative density) is the ratio of the density of a substance to the density of a reference substance.
![]()
where:![]()
![]()
![]()
![]()
5. Bulk Modulus of Elasticity
Bulk modulus of elasticity measures a fluid’s resistance to compression. It quantifies how much pressure is required to produce a given fractional change in volume.
A high bulk modulus means the fluid is difficult to compress (stiff). Liquids have high bulk moduli and are often treated as incompressible, while gases have low bulk moduli and compress easily.
Critical for water hammer analysis, acoustic wave propagation, hydraulic system design, and understanding fluid compressibility effects.
Example
A liquid compressed in a cylinder has a volume of 1000 cu.m at ![]() and a volume of
and a volume of ![]() at
at ![]() . What is its bulk modulus of elasticity?
. What is its bulk modulus of elasticity?
6. Compressibility
Compressibility is the reciprocal of the bulk modulus. It measures how much a material decreases in volume when subjected to pressure.
![]()
Types of Compressibility
Isothermal Compressibility
Volume change at constant temperature
Adiabatic Compressibility
Volumes changed without heat transfer
Example
If the Bulk Modulus of Water is 2.2 GPa. What is its coefficient of Compressibility?
7. Viscosity
Viscosity is defined as the fluid’s resistance to flow. It represents internal friction between fluid layers moving at different velocities.
Dynamic Viscosity
Units: poise or 0.1 Pa-s
where:![]()
![]()
![]()
Viscosity arises from intermolecular forces and momentum exchange between fluid layers. Higher viscosity means more resistance to flow, honey has high viscosity, water has low viscosity. Viscosity generally decreases with temperature for liquids and increases with temperature for gases.
Kinematic Velocity
![]()
where:![]()
![]()
![]()
While dynamic viscosity relates shear stress to velocity gradient, kinematic viscosity relates to how quickly momentum diffuses through the fluid. It’s particularly useful because it combines both viscous and inertial effects.
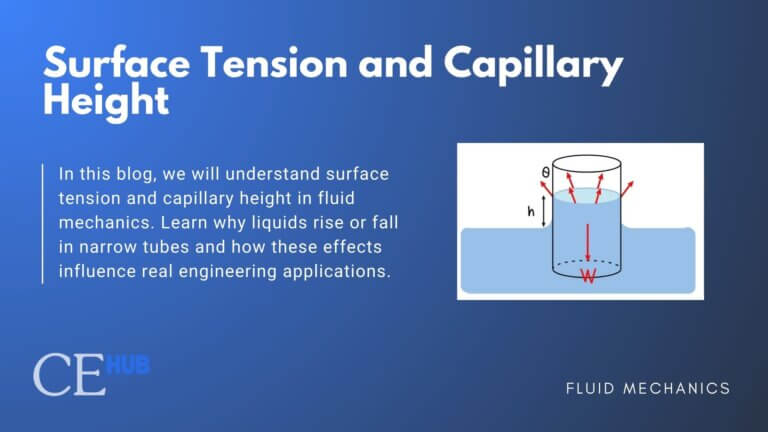
8. Surface Tension
The force responsible for the tension that acts along its surface and arises from the attractive forces between the liquid’s molecules. The strength of this force per unit length is known as surface tension.
![]()
Where: ![]()
9. Capillary Action
is when liquid rise or fall through narrow spaces without external forces.
![]()
Where:![]()
![]()
![]()
![]()
![]()
Capillary action was most used in applications like Porous media flow, soil moisture movement, oil recovery, chromatography, heat pipes and inkjet printing.
References
J. D. Anderson Modern Compressible Flow with Historical Perspective, 3rd ed. New York: McGraw-Hill, 2003.
F L U I D M E C H A N I C S FUNDAMENTALS AND APPLICATIONS Third Edition. (n.d.). https://engineeringbookslibrary.wordpress.com/wp-content/uploads/2019/03/fluid-mechanics-fundamentals-and-applications-3rd-edition-cengel-and-cimbala-2014.pdf
📍This post is part of FLUID MECHANICS COMPLETE Lessons. Also, if you want to avail the complete Cheat Sheet just click the “shop now” below.
Fluid Mechanics and Hydraulics Cheat Sheet
Master the fundamentals of fluid mechanics with this complete Fluid Mechanics Cheat Sheet designed for civil engineering students and exam reviewers. This digital guide summarizes the most important formulas, concepts, and equations. Not only it is aesthetic but also categorized by topics.